Address
304 North Cardinal St.
Dorchester Center, MA 02124
Work Hours
Monday to Friday: 7AM - 7PM
Weekend: 10AM - 5PM
The IICC has collaborated with several government bodies and industry organizations to develop two standards that together allow for true Plug & Play connectivity of IVD instruments to Middleware and Laboratory Information Systems (LIS):

The IICC has collaborated with several government bodies and industry organizations to develop two standards that together allow for true Plug & Play connectivity of IVD instruments to Middleware and Laboratory Information Systems (LIS):
The IICC has collaborated with several government bodies and industry organizations to develop two standards that together allow for true Plug & Play connectivity of IVD instruments to Middleware and Laboratory Information Systems (LIS):
The LAW Profile defines the physical connection, message definitions (based on the HL7 Messaging Standard v2.5.1), and workflow definitions between instruments, middleware, and LIS systems in the laboratory. IICC collaborated with the IHE Pathology and Laboratory Medicine (PaLM) domain to develop the LAW Profile. LAW is currently being implemented by all major IVD companies.
Defines the digital publication of LOINC using vendor defined IVD tests associated with a set of predefined LOINC codes. LIVD assures that laboratory personnel select the appropriate LOINC codes for IVD test used by their laboratory. It also allows LIS systems to automatically map the correct IVD vendor test result to a LOINC code.
The LAW Profile and LIVD specifications should have a significant positive impact on laboratory operations. Clinical laboratories are encouraged to ask their instrument, middleware, and LIS vendors about their current or planned support for the IICC/IHE Laboratory Analytical Workflow (LAW) and LIVD. The LAW Profile is currently being implemented by all major IVD companies.
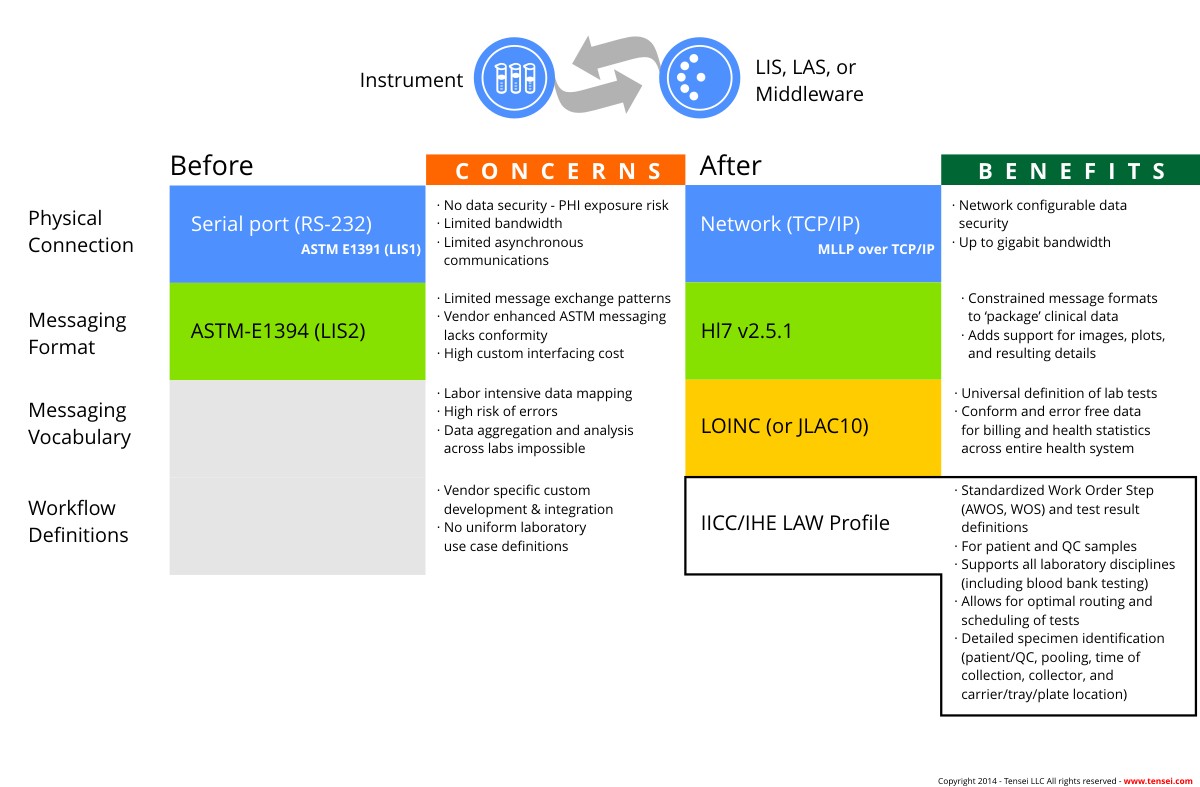
Clinical Laboratory Connectivity before and after LAW and LIVD
