Address
304 North Cardinal St.
Dorchester Center, MA 02124
Work Hours
Monday to Friday: 7AM - 7PM
Weekend: 10AM - 5PM
The IVD Industry Connectivity Consortium (IICC) is a global, nonprofit organization dedicated to creating and encouraging adoption of a unified connectivity standard to reduce the cost and variability of data exchange between IVD devices and healthcare informatics. This will improve healthcare efficiency and patient care. Member organizations include Abbott Diagnostics, Beckman Coulter, Becton Dickinson, bioMérieux, Data Innovations, Orchard Software, Ortho Clinical Diagnostics, Roche Diagnostics, Siemens Healthcare Diagnostics, and Systelab Technologies, S.A.
“The potential to reduce the cost and time involved with lab device connectivity has been demonstrated with the IHE LAW Profile. As an industry, it’s time we move beyond our interoperability challenges and refocus our energy on advancing healthcare.”
Eric Olson – President Emeritus, IICC, and Vice President, Portfolio & Product Management, Siemens Healthcare Diagnostics, Inc.
“The publication of the LAW Profile as a supplement for trial implementation and the successful completion of the IHE European Connectathon 2012 are important milestones. The next step is implementation in the coming generation of IVD products, which will integrate the clinical lab into the mainstream of healthcare information flow.”
Edwin O. Heierman – Chief Technology Officer, IICC, and Informatics Software Architect, Abbott Diagnostics Division
“Over the next 5 years, Meaningful Use will have a huge impact on the IVD world, and all stakeholders will benefit—especially the patients. Now is the time to act. We want to drive the change, not just sit back and let change drive us.”
Jay B. Jones, PhD, DABCC – Board Member and Chair of the Provider Review Committee, IICC, and Director, Chemistry & Regional Labs, Geisinger Health System
“The incorporation of the LAW Profile allows for simplification and standardization across different analytical instruments. The challenge will be to continue to improve the pace of laboratory processes by means of such innovations to reduce costs, improve quality and provide better care for patients.”
Patrick Collyer – Systelab Technologies, S.A.


Emery Stephans’ role in developing the standard began when he was appointed as the first chair of the AACC Point of Care Testing Division’s Industrial Liaison Committee. With the appointment, he was given the charge of developing a connectivity standard. Initially this was thought to be more of a consciousness-raising effort than a truly achievable objective. Fortunately, Emery did not see it this way and proceeded to identify and obtain support from several individuals who had participated in development of the HL7 protocol for medical devices. Emery found industrial partners to provide the organizational and financial support needed to create an ad hoc organization for developing the standard. This organization became known as the Connectivity Industry Consortium (CIC). Emery was also heavily involved in providing various types of support as the CIC matured and created the connectivity standard. Although there were many individuals whose contributions were critical to the success of the effort, Emery’s role was clearly the largest and most pervasive. He provided the spark that got the effort going and the glue that held it together.
Emery did not stop at the POCT standard, which is now available from CLSI. He expanded the effort from POCT devices to the entire clinical laboratory. We are very grateful for his leadership and vision and his considerable impact on improving the practice of laboratory medicine.
Emery is the CEO and founder of Enterprise Analysis Corporation. It was Emery’s passion for healthcare and science that prompted him to start EAC after 26 years at IBM.
In February 2009, following founding and appointment of the leadership team, the IICC Technical Team was formed; a project plan was approved; and the task of modernizing lab connectivity began. Our path was clear: to build upon available technology and standards, to draw on the expertise and experience of existing standards organizations, and to define the scope of work relative to the types of data, types of IVD testing and types of IT systems to be addressed.
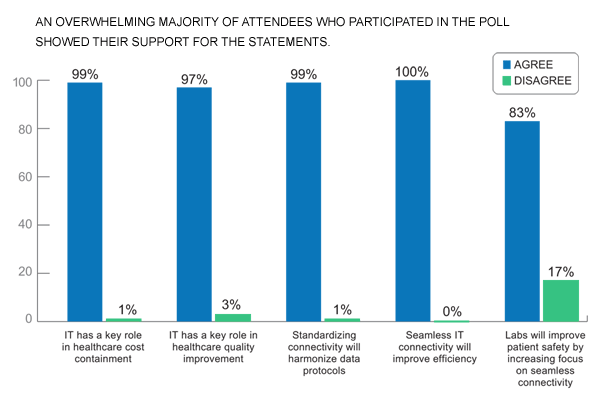
To assess the IVD community’s sentiment about key issues related to lab connectivity, IICC conducted a poll at the 2010 AACC Annual Meeting in Anaheim, CA. Attendees were asked whether they agreed or disagreed with the following statements: